COV Iran Barekat
COV Iran Barekat is a COVID-19 vaccine developed in Iran by Shifa Pharmed Industrial Group, a subsidiary of the Barkat Pharmaceutical Group. It is an inactivated virus-based vaccine.Iranian authorities have authorized its emergency use. This makes it the first locally developed COVID-19 vaccine to be approved for emergency use in the Middle East.
Officials in charge say they are in the process to publish the results of the clinical trials in a peer-reviewed journal. The interim results of the phases 1 and 2 trials showed 93.5% (95% CI, 88.4–99.6%) of the receivers of the vaccine have produced neutralizing antibodies against SARS-CoV-2. Those results have not been peer-reviewed yet and describe the immunogenicity of the vaccine and not its efficacy.
Multiple Iranian personalities have received the vaccine, including the Supreme Leader, Ali Khamenei and the President, Ebrahim Raisi. Up to 10 October 2021, about 16 millions doses have been produced according to Shifa Pharmed’s CEO.
Medical uses
It is given by intramuscular injection and requires two doses given 28 days apart.
Pharmacology
COVIran Barekat is an inactivated virus-based vaccine. In other words, “it is made of a coronavirus that has been weakened or killed by chemicals, similar to how polio immunizations are made”.
Developers
The Execution of Imam Khomeini’s Order (EIKO) and Barkat Pharmaceutical Group is parent company of Shifa Pharmed Industrial Group. It’s reported to be “state-affiliated”. Shifa Pharmed products include seven drugs and three biologics besides their COVID-19 vaccine which is their first vaccine to be produced. Around 650 people worked in three shifts, around the clock, to develop the vaccine.
Dr. Minoo Mohraz has been selected as the lead of the “Corona vaccine project in Iran”. Mohraz is an Iranian physician, scientist, and AIDS specialist. She is a Full Professor (Emeritus) of Infectious Diseases at Tehran University of Medical Sciences and head of the Iranian Centre for HIV/AIDS. Mohraz has also served, within the World Health Organization, as an expert on HIV/AIDS in Iran and the Eastern Mediterranean.
Manufacturing
First production line, March 2021. Barkat Pharmaceutical Group started constructing a factory for vaccine production on 17 December 2020 with the goal to build it in 3 months. According to Execution of Imam khamenei’s Order (EIKO), under the direct control of the Supreme Leader of Iran, “production of the vaccine developed by one of its companies, Shifa Pharmed, could reach 12 million doses per month, six months after a successful trial ends”.
Mass production began on 15 March 2021. The first produced batch was unveiled on 10 May 2021.
There are three production lines for COVIran Barekat.
Cumulate production capacity will be 25-30 million doses per month when all lines will start producing.
The first line has a capacity of 3-4 million doses per month and started producing as of 15 March 2021. The second line has a capacity of 6-8 million doses per month. On 17 August, it was in the process of producing its first batch. The third line has a capacity of 16-20 million doses per month. On 17 August, 95% of its equipment were reported to be prepared and installed and officials in charge aimed to begin its operation as of end of September.
Up to 3 July 2021, about 2.7 millions doses of COVIran Barekat vaccine have been produced and 400,000 doses have been delivered to the Iranian Ministry of Health, according to Mohammad Mokhber, the Deputy Coordinator of the Execution of Imam Khomeini’s Order.
Up to 27 July 2021, about 5 millions doses have been produced and 1.3 million delivered to the Ministry of Health, according to Mokhber.
Up to 30 August 2021, about 8 millions doses have been produced and 4.2 millions delivered to the Ministry of Health, according to EIKO.
Up to 10 October 2021, about 16 millions doses have been produced and 7.5 millions delivered to the Ministry of Health, according to Shifa Pharmed’s CEO.
Preclinical trials
The results of the preclinical study conducted on animals have been published in a preprint (not peer reviewed) on 10 June 2021. According to the developers of the vaccine, trials showed that the vaccine was safe and effective in animals.
On 26 October 2021, the results of the preclinical study have been published in the peer-reviewed Journal of Medical Virology. Clinical trials Iran’s first domestic COVID-19 vaccine candidate started clinical trials in late December 2020. Vaccines for clinical trials.
Clinical trials are tests done in humans, to see whether a treatment is safe and effective. There are different phases of clinical research, and each phase has its own primary focus. For example, the focus of Phase I clinical trials is basic safety and dose information.
All studies were randomized, double-blind, parallel arms, placebo-controlled and have been conducted on healthy volunteers.
Phase I
Iran Food and Drug Administration has approved the vaccine for testing on humans. According to reports, more than 65,000 Iranians volunteered to test the vaccine while only 56 people were needed.
On 29 December 2020, human trials of Iran’s first domestic COVID-19 vaccine candidate were started. The first volunteer who received a shot of COVIran Barekat was Tayyebeh Mokhber, the daughter of Mohammad Mokhber, director of Setad. Minister of Health Saeed Namaki and Vice President for Science and Technology Sorena Sattari participated at the ceremony of vaccine injection.
Phase 1’s primary outcome was safety assessment. A first phase 1 study was conducted on 56 healthy volunteers who were at the age of 18–50. Last injections of the second doses occurred on 4 March 2021. A second phase 1 study was conducted on 32 volunteers aged 51-75 years. First injections occurred on March 15 and last injections on April 9.
Phase II/III
Phases II and III of the clinical trials were combined, allowing to start the third phase before the second completed. Participants were aged between 18 and 75 years.
Phase 2’s primary outcome was immunogenicity assessment. It was conducted on 280 volunteers. The first volunteers were inoculated on March 15 and the last injections of the second doses occurred on May 25.
Phase 3’s primary outcome was efficacy assessment on preventing mild, moderate and severe disease. It was conducted on 20,000 participants across 6 cities in Iran (Tehran, Karaj, Shiraz, Isfahan, Mashhad and Bushehr). Phase III has been reached on April 21; and the first injections occurred on April 25. Up to June 14, 18,000 volunteers received their first dose and 2,000 received their second dose of the vaccine or placebo. Dr. Minoo Mohraz was the first volunteer of the phase 3 that received the vaccine.
Results
On 16 June 2021, a summary of the results obtained in the phase 1 and phase 2 of the clinical trials have been published in the press. According to the report, only mild adverse effects were registered execept one case of hypotension, one case of level-2 headache and one case of diminution of platelets that didn’t need medical care. Conventional Virus Neutralizing Test (cVNT) is reported to have shown 93.5% immunogenicity (95% confidence interval: 88.4 – 99.6%). On 23 June 2021, the production project manager of the vaccine stated that the results of the second phase have shown “the serum of the people who received the vaccine has 93.5% power to neutralize the virus; thus meaning the vaccine has a very good efficacy that will be shown after the end of the third phase”.
There are claims of some issues with the vaccine’s scientific documentation article according to the U.S. Agency for Global Media-owned broadcasting network Radio Farda,
On 27 July 2021, the Director of the Barkat Pharmaceutical Group stated that a paper with the result of the clinical trials have been prepared and submitted. “It will be sent to 10 important scientific journals of the world but it may take time before they publish it”.
In February 2021 (while phase 1 study was ongoing), the head of the vaccine production team at the Setad stated that the vaccine also neutralizes the British mutated COVID-19 virus. Trial on children aged 12-18
A phase I-II clinical trial on children aged 12-18 years began on 23 November 2021. It consist of a comparison study where half of the 500 participants receive a Coviran Barkat vaccine and the other half a Sinopharm BIBP vaccine. Primary outcome is safety and immunogenicity assessment.
Other countries
As official in charge of manufacturing Iran Barekat vaccines, Mohammad Reza Salehi said, “some neighboring countries tend to enter the third phase of the clinical trial of the Iranian COVIran Barekat”. They are reviewing recommendations to let them participate.
Authorizations
See also: List of COVID-19 vaccine authorizations § COVIran Barakat
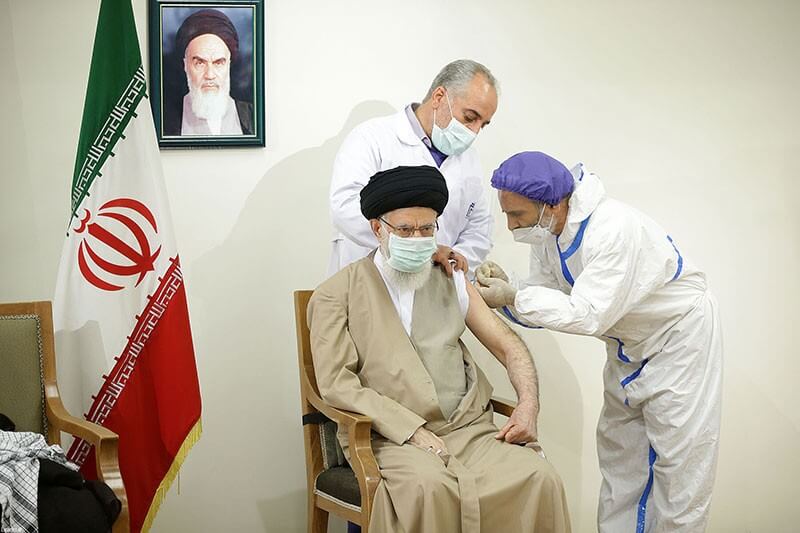
The vaccine received its authorization license from the Iran Food and Drug Administration on June 13, 2021. It is now in the process to be registered by the World Health Organization. Society and culture The Supreme Leader of Iran, Seyyed Ali Khamenei receiving his first dose of COVIran Barekat on 25 June 2021.
Ali Khamenei, the Supreme Leader of Iran, have received his first dose of Iran’s locally made COVIran Barekat vaccine on 25 June 2021 (twelve days after it received its public use authorization from Iranian authorities). He received his second dose on 23 July 2021.
Ebrahim Raisi, Mohammad Bagher Ghalibaf, Ali Larijani, and Amoli Larijani, have been vaccinated with COVIran Barekat.
Source:
https://en.wikipedia.org/wiki/COVIran_Barekat#History
[/av_textblock]